Back in 1913, a Nobel laureate pioneered the idea of "Magic Bullets": if a toxic drug (i.e., a bullet) is mounted on a carrier that specifically targets tumor cells, it may be possible to precisely kill cancer cells without harming normal cells. However, this idea was far beyond the technological capabilities of the time.
Since then, with the advancement of monoclonal antibody technology, the world's first
antibody-drug conjugate (ADC) finally came into being in 2000 after the unremitting efforts of scientists.
Chemenu is a professional supplier of pharmaceutical intermediates and APIs, please visit our website for more products. We will introduce you ADC drugs and their development trend.
ADC track is on fire
In 2017, Mylotarg was relaunched, together with the FDA's approval of the first new CD22 ADC drug on the market, making the ADC track warm up again. 2019, the FDA followed with the approval of three new ADC drugs, in addition to the famous DS8201, the other two are new target ADCs targeting CD79b and Nectin-4, completely inspiring the industry to ADC drug investment in research and development enthusiasm, a series of billions of dollars, tens of billions of dollars transactions occur frequently, small-scale ADC project transactions are countless.
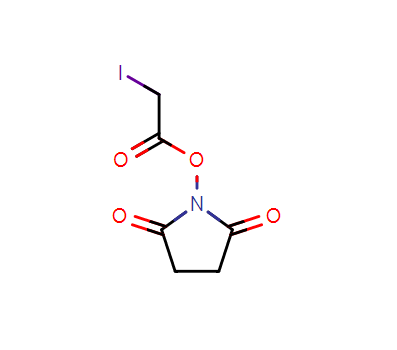
Product Name:SIA Crosslinker
IUPAC Name:2,5-dioxopyrrolidin-1-yl 2-iodoacetate
CAS:39028-27-8
Chinese companies related
With more new target ADC drugs approved for marketing, a new chapter has opened in the development competition in the ADC track. On the one hand, more and more ADCs with similar targets are entering the clinic or advancing to the late stage of the clinic, and the differentiated indication development strategy and clinical design determine the success or failure of the commercialization of ADC projects. On the other hand, more and more ADC projects are entering the late stage of clinical development or about to submit marketing applications, and large-scale commercial production becomes an issue that companies need to consider and lay out in advance.
Given the special nature of ADC drugs, whether self-built or outsourced, the formation of complete commercial production capacity is not a one-time or one-sided process. In order to effectively and reasonably promote product development and marketing plans, commercialization production capacity must be laid out as early as possible, and the selection of CDMO partners is also an important strategy in the current market environment.
Product Name:Maytansinoid DM4
IUPAC Name:(1S,3S,5S,6S,16Z,18Z,20R,21S)-11-chloro-21-hydroxy-12,20-dimethoxy-2,2,5,9,16-pentamethyl-8,23-dioxo-4,24-dioxa-9,22-diazatetracyclo[19.3.1.1¹⁰,¹⁴.0³,⁵]hexacosa-10,12,14(26),16,18-pentaen-6-yl (2S)-2-(N,4-dimethyl-4-sulfanylpentanamido)propanoate
CAS:799840-96-3
Chemenu has both wholly owned and partnered facilities to meet pilot and commercial needs. ISO 9001: 2015 is the basis of our product and service quality management system. In order to meet higher requirements, we follow additional quality standards: Good Manufacturing Practice (GMP) regulations. You can
contact us for more information.
.png)